Water is one of the most important substances on Earth. It is essential for life. We drink it, use it for cooking, and clean with it. But have you ever wondered what water is made of? In this article, we will explore the basic chemistry of water, focusing on how many hydrogen atoms are in a molecule of water.
The Composition of Water
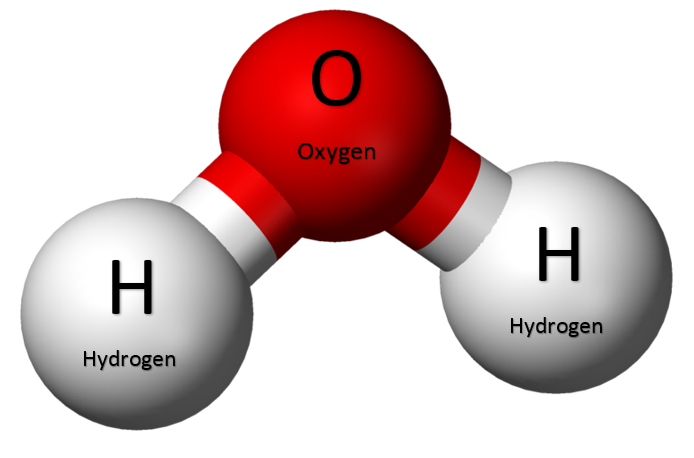
Water is a chemical compound. This means it is made up of two or more different elements. The chemical formula for water is H₂O. This simple formula tells us a lot about water’s composition. The “H” stands for hydrogen, and the “O” stands for oxygen.
Understanding Chemical Formulas
To understand how many hydrogen atoms are in a water molecule, we need to understand chemical formulas. A chemical formula uses symbols to represent the elements in a compound. It also uses numbers to show the amount of each element. In H₂O, the number 2 after the H tells us that there are two hydrogen atoms. The O has no number after it, which means there is one oxygen atom.
Counting Hydrogen Atoms
Now that we understand the chemical formula, we can answer the question. How many hydrogen atoms are in a molecule of water? The answer is two. Every molecule of water has two hydrogen atoms and one oxygen atom.
Why Two Hydrogen Atoms?
The reason there are two hydrogen atoms in a water molecule has to do with how atoms bond. Atoms bond to achieve a full outer shell of electrons, which makes them stable. Oxygen has six electrons in its outer shell but needs eight to be stable. Hydrogen has one electron and needs two to be stable.
When two hydrogen atoms bond with one oxygen atom, they share electrons. This sharing allows both hydrogen and oxygen to achieve full outer shells. The oxygen atom shares one electron with each hydrogen atom, and each hydrogen atom shares one electron with the oxygen atom. This creates a stable molecule.
The Shape of a Water Molecule
The structure of a water molecule is also important. Water molecules have a bent shape. This shape is due to the way the hydrogen atoms attach to the oxygen atom. The two hydrogen atoms bond to the oxygen atom at an angle. This angle is about 104.5 degrees. This bent shape is what gives water its unique properties, such as its ability to dissolve many substances.
Properties of Water
Water has many unique properties that make it vital for life. One of these properties is its ability to act as a solvent. A solvent is a substance that can dissolve other substances. Water’s bent shape and polar nature (having a positive and a negative side) allow it to dissolve many different substances. This makes water an excellent medium for chemical reactions in our bodies.
Water also has a high specific heat capacity. This means it can absorb a lot of heat before it gets hot. This property helps regulate the Earth’s climate and keeps our bodies at a stable temperature.
The Importance of Water in Biology
Water’s ability to form hydrogen bonds makes it essential for life. Hydrogen bonds are weak bonds that form between the positive side of one water molecule and the negative side of another. These bonds give water its unique properties, such as its high surface tension and ability to form droplets.
In biology, water is crucial for many processes. It helps transport nutrients and waste in and out of cells. It also plays a role in regulating body temperature. Additionally, water is a key component of blood, which carries oxygen and nutrients to our cells.
Water in the Environment
Water is not only important for living organisms but also for the environment. It covers about 71% of the Earth’s surface. Oceans, rivers, and lakes are all vital parts of the Earth’s water cycle. This cycle involves the continuous movement of water on, above, and below the surface of the Earth.
Water evaporates from the surface of oceans and lakes, forms clouds, and falls back to Earth as precipitation. This cycle helps to distribute heat around the planet and supports all forms of life.
Water and Human Use
Humans use water in many ways. We drink it to stay hydrated. We use it in agriculture to grow crops. Industries use water to manufacture products. Water is also used for recreation, such as swimming and boating.
Access to clean water is crucial for health. Contaminated water can cause serious illnesses. This is why water treatment plants are important. They help remove harmful substances from water to make it safe to drink.
Water Conservation
Given its importance, conserving water is essential. Many parts of the world face water scarcity. This means there is not enough water to meet all the needs. Simple actions can help conserve water. For example, fixing leaks, taking shorter showers, and using water-efficient appliances can make a big difference.
Conclusion
Water is a simple molecule with two hydrogen atoms and one oxygen atom. Despite its simplicity, it is vital for life on Earth. It has unique properties that support biological processes, regulate the environment, and serve human needs. Understanding the composition and properties of water helps us appreciate its importance and encourages us to use it wisely. So next time you drink a glass of water, remember that it is made up of two hydrogen atoms and one oxygen atom, working together to support life.